Remember that strange smell when your grandma was making your favourite biscuit? Its source must have been the Ammonium Bicarbonate or “Hartshorn”, used for the baking process, and the smell had disappeared by the time the cake was ready. So why is salt volatile so special that it is worth putting up with the awful smell while baking?
This article will help you with the following questions:
- What is Ammonium Bicarbonate (Hartshorn)?
- Does Ammonium Bicarbonate have any side effects?
- What cakes can we use salt volatile for?
- The difference between Ammonium Bicarbonate, baking powder and Baking Soda.
What is Ammonium Bicarbonate (Hartshorn)?
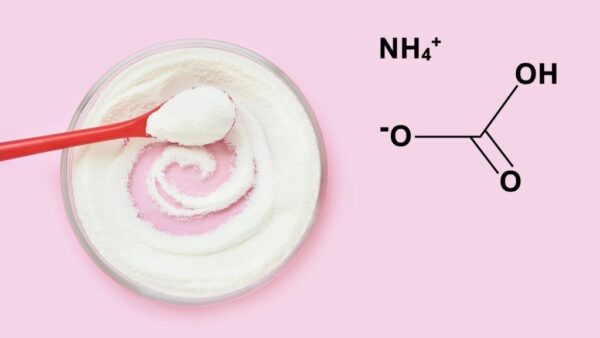
Ammonium Bicarbonate or Hartshorn can also be called ammonium hydrogen carbonate; its chemical symbol is NH4HCO3. It is labelled with E503ii in the food industry. It is produced through chemical reactions from carbon dioxide and ammonia. At room temperature, salt volatile is white, crystalline powder. At high temperatures, for instance, during baking, it dissolves into carbon dioxide, water, and ammonia. This is why we barely notice the smell of ammonia when kneading the dough, but gas production starts vigorously in the hot oven. Ammonium Bicarbonate increases the volume of the dough – this is the purpose of using this substance.
Ammonium Bicarbonate has a long history: it was invented by August Oetker at the end of the 1800s for loosening the texture and increasing the volume of cakes. During that time, baking powder as we know it today did not exist. Today, Ammonium Bicarbonate is rarely used for baking due to the pungent smell; they often replace it with baking powder.
Does Ammonium Bicarbonate have any side effects?
Salt volatile is a harmless food ingredient; there is no prescribed or recommended limit of its daily consumption, and there are no known side effects either. It may irritate our eyes and respiratory system during the production of gas, so we should pay attention to air the kitchen thoroughly when using it.
What cakes can we use salt volatile for?
- It is perfectly suited to use for thin dough and cakes with several layers, as thicker dough might retain the smell of ammonium
- The molecules of Ammonium Bicarbonate only start dissolving in the hot oven and not at room temperature, so it is ideal for dough that has to be rested for a long time before baking.
- After baking, the dough made with Ammonium Bicarbonate becomes brittle, so we must be careful with it until it is softened by the cream filling.
The difference between Ammonium Bicarbonate, baking powder and Baking Soda?
All three additives are responsible for the soft and airy texture but are not perfectly interchangeable with one another. Each ingredient has its own purpose and advantage in the kitchen.
- Baking powder should not be used for any dough that has to be rested before baking. After mixing it with flour, we should start baking as soon as possible to prevent the dough from collapsing. In the case of baking powder, the expiration date is also very important.
- Baking soda can take full effect in the dough only if used in an acidic medium, so it is recommended to use acidic ingredients such as lemon, vinegar, honey, or other acidic substances.
- Ammonium Bicarbonate is the perfect raising agent for thin dough that needs to be rested for a long time before baking, as well as for layer cakes, gingerbread, and biscuits. Don’t forget to air the kitchen while baking!
Salt volatile, the acidic agent that served our grandma so well, is still a very useful additive these days. If you learn how to apply it and can endure the pungent smell while baking, you can surprise the family with an authentic six-layer cake during the holidays ‒ just like in the old times!